Air pollution is the contamination of air by harmful substances that can cause health problems. These can include burning eyes and nose, an irritated throat, breathing problems, and even premature death. Air pollution can also have a negative effect on the environment.
Some air polluting chemicals are explained below:
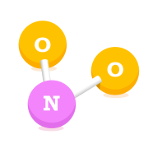
Nitrogen Dioxide (NO2)
Nitrogen Dioxide is created when nitric oxide meets oxygen in the air, this causes a reaction which creates Nitrogen Dioxide. The highest NO2 levels occur where there is heavy traffic e.g cars and other vehicles.
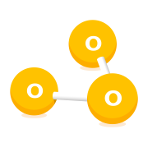
Ozone (O3)
Ozone is a naturally occurring gas in the upper layers of the atmosphere. However at ground level sunlight reacts with other pollutants to create ozone.
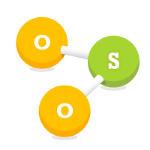
Sulphur Dioxide (SO2)
Sulphur dioxide is produced when a material, or fuel, containing sulphur is burned. It is a gas associated with industrial areas, in particular industrial processes such as the burning of fuels (coal and oil). A natural source of sulphur dioxide is emitted by volcanoes.
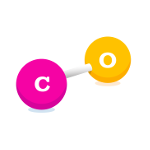
Carbon Monoxide (CO)
Carbon Monoxide is a colourless, odourless poisonous gas produced by incomplete, or inefficient, combustion of fuel. It is predominantly produced by road transport, in particular petrol-engine vehicles.
Household boilers and cookers can be sources in the home and CO alarms can be just as important as smoke alarms – they could save your life.

Particulate Matter
Particulate Matter is made up of a wide range of particles, some occur naturally while others are created. Naturally occurring particulates are sand and sea salt, while others are created by chemical reactions in the atmosphere or emitted through traffic emissions.
